Managing the Impact of the Drug Pipeline on Costs
October 16, 2023
Josh SimermanPharmaceutical companies aim to provide treatments to manage pain, symptoms, and side effects, and improve patients' quality of life, allowing them the freedom to live healthier and more fulfilled lives. Pfizer, a global pharmaceutical company, pointedly uses as its sole Purpose Statement “Breakthroughs that change patients’ lives.” While this ultimate objective gets muted when corporate revenue and profits are weighed into the discussion, the doctors and scientists working are focused on the clinical results. The cost of their work and the products they develop get compounded as they pass through the health care system and ultimately falls on patients, insurers, and employers to truly manage. How to control this cost and who is responsible for it continues to be debated by developers, users, payers, and regulators, with no end in sight.
The median annual price of the 17 novel drugs (new substances not previously identified) the U.S. Food and Drug Administration (FDA) approved in 2022 was $222,000. This is up from $180,000 in 2021, or nearly 23%. The price range for a single treatment of those drugs is $27K to $3.5M. The drug pipeline continues to expand and remain full, with more than 1,000 cell-, gene-, and tissue-based therapies in development globally. Preparing for continued volume and cost expansion needs to remain top of mind.
For employers to adequately protect their plan and members from fully absorbing these high costs, they need to understand the drug pipeline’s impact on costs and how health insurers react to that as they prepare for 2024.
Drug Pipeline and Cost Influencers
Drug manufacturers must continue developing new treatments to impact new diseases and further existing treatments to achieve such a lofty purpose statement. This creates a drug pipeline, which refers to the process of developing and bringing new medications to market. The impact of the drug pipeline on cost can be evaluated from a few perspectives:
- Research and Development (R&D) Costs: Developing a new drug involves significant investment in research, testing, and clinical trials. Pharmaceutical companies usually bear these costs, contributing to the overall expenses associated with drug development. The greater the R&D costs, the greater the final cost of the treatments.
- Market Competition: As new drugs are introduced, they are often targeted to compete against existing treatments. This competition can lead to price fluctuations, as pharmaceutical companies may adjust the price of existing treatments to remain competitive and retain market share. While new drugs are commonly released at higher prices, in some cases, introducing new drugs may result in lower costs due to increased competition. Biosimilars, which are generic versions of specialty drugs that have a more complex molecular structure, have the potential to continue to drive prices down for the end-user, the patient, and the insurer.
- Access to Innovative Therapies: The drug pipeline is crucial to expanding treatment options for various diseases and conditions. By continuously bringing new drugs to market, the pipeline enhances access to innovative therapies. Gene therapy drugs are one class of drugs that repair defective or replace missing genes in cells to correct genetic disorders. Up to 90 new gene and cellular therapies could be approved by 2031, representing $30 billion in healthcare spending. While these advancements may come at a cost, they also provide patients with potentially lifesaving or life-improving treatments that were previously unavailable.
- Health Insurance Coverage & Government Regulation: The cost of drugs can also be influenced by health insurance coverage and state and federal policies. Insurance companies negotiate prices with pharmaceutical manufacturers, impacting patients’ final out-of-pocket costs. Additionally, government policies and regulations may affect drug pricing and access, particularly when it comes to essential or high-demand medications. Continued transparency legislation shines a light on drug pricing by requiring manufacturers and other supply chain entities to provide information on drug pricing. Both of their goals are to moderate price increases over time.
It's important to note that the drug pipeline and its impact on cost are complex and multifaceted. The interplay between research, development, market dynamics, and healthcare policies contributes to the final cost of medications and provides concern for continued growth in the short and long term.
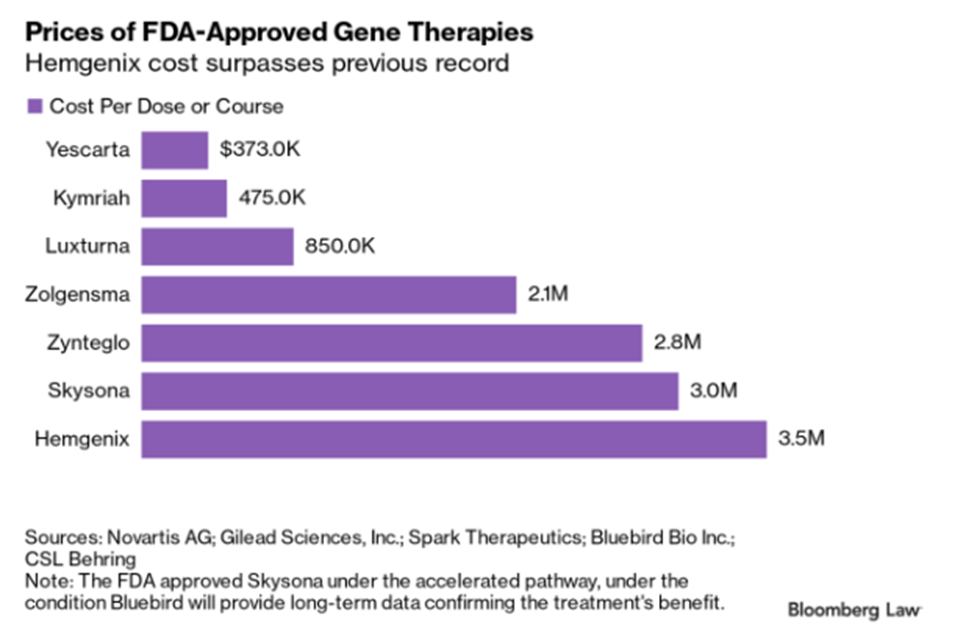
Hemophilia Gene Therapy in a Vacuum
Specialty drugs have had a significant impact on the treatment of hemophilia. Hemophilia is a rare genetic bleeding disorder characterized by the inability of the blood to clot properly. Prior to the development of gene therapy drugs, treatment options were limited, and patients often relied on regular infusions of clotting factor concentrates derived from human plasma.
The introduction of a new drug, HEMGENIX revolutionized hemophilia treatment in 2022. HEMGENIX uses a gene therapy approach called gene transfer. This approach aims to introduce a working gene into the body to produce functional factor IX and support natural blood-clotting ability. The impact of specialty drugs on hemophilia has been positive in the following ways:
- Improved clotting factor supply by reducing the volume needed for patients
- Enhanced safety by lowering the risk of transmitting blood-borne infections
- Increased patient convenience and flexibility by reducing treatment times and cycles
- Deployment of personalized medicine using genetic testing and personalized dosing to tailor treatment regimens to individual patients, optimizing therapy and minimizing complications
In a vacuum, innovation in the hemophilia drug treatment space is a fantastic success that anyone who qualifies for the available drug treatments should be able to obtain.
The drug manufacturer’s objective of achieving a successful improvement in patient outcome is met, but at an unknown cost by an unknown party. In the case of HEMGENIX, that one-time cost is a $3.5 million treatment, in contrast to the potential cost of a lifetime of existing treatments that typically range from $1 to $5 million. While this is a job well done by the pharmaceutical company and the result is great for the patient, how insurers approve, manage, and absorb these costs is still being sorted out.
Insurer Reactions
To address these cost concerns, some insurance companies are taking interesting steps to help protect their profitability and options for their corporate clients. Some firms are creating and/or joining collective purchasing agreements. Alternatively, some insurers are tying payment for the drugs (to the manufacturer) with the treatment’s results.
Insurers have also set conditions to limit who can receive gene therapies to strike a balance between providing patients with access to gene therapies and managing their costs. Some Pharmacy Benefits Managers search for the most optimal specialty drug distribution point. Perhaps it is a small specialty pharmacy that only handles certain types of medications, but they can be mailed to the patient’s home far cheaper from that boutique pharmacy versus the big-name, large-store pharmacies.
Additional challenges with the insurers: pharmacy rebates are plentiful, especially for the more expensive medications. The insurance company often retains these. For example, a biosimilar drug might be new and cost less than a big-name medication that is currently heavily prescribed. There is a chance that the insurance company is not motivated to incentivize the biosimilar drug (on its formulary) because the rebate is too low compared to the big-name medication’s rebate, and profitability is still a key metric for the insurance companies.
Employer Response
The Business Group on Health, representing 100 larger employers, released its annual 2024 Large Employer Health Care Strategy Survey in August 2023 and found that 91% are either concerned or very concerned about the overall pharmacy cost trend. Major concerns included the lack of transparency around rebates negotiated by pharmacy benefit managers, the complexity of the supply chain, and the barriers for employers who want to audit the performance of the players in that supply chain, according to the survey.
Employers that self-fund their health coverage can exclude cell and gene therapies, although they can run the risk of negative employee perspectives on not having access to medications. However, restricting new-to-market medications from being covered on the formulary is a way to prevent cost increases for new specialty medications (at least temporarily). Most commonly, ongoing implementation of plan design changes, coverage limits, and drug access controls to address costly medications and treatments are being planned by employers for 2024. Like some of the insurer’s responses, more advanced self-funded employers are also contemplating partnering with boutique, transparent pharmacy benefit managers and risk pooling for gene therapy drugs.
Conclusion
As drug innovation continues, the cost of drugs will rise, and with whom that financial burden lies is going to continue to be debated. While the drug manufacturers, insurers, and the government work through those delicate conversations, patients who could benefit from new specialty drugs will also be voicing their desires. This leaves employers holding the checkbook as the key financier of medical and pharmacy benefit costs and insurance. The expectation is that employer’s concerns will continue to increase, as will their efforts to drive change through controlling their business costs and lobbying regulators. In the meantime, employers are partnering with benefit consultants, insurance companies, pharmacy benefit managers, and new market entrants that are most effectively contracting and managing specialty drugs, which is imperative to the sustainability of affordable coverage.
Effective management can be a short- and mid-term solution, but without real change in the marketplace, the pharmaceutical industry’s purpose to change patient lives is in jeopardy.
Various Sources:
https://www.pharmavoice.com/news/gene-therapy-cost-payment-OHE/643659/
Magellan Rx Report | Magellan Rx Management
https://www.evernorth.com/our-solutions/embarc-benefit-protection
https://www.vantagemarketresearch.com/industry-report/hemophilia-market-1216


